Traveling within the World
Linking your favorite traveling artists across the globe
Forging, once and for all! by Kevin R. Cashen, www.cashenblades.com
The �lowdown� on smithing
Anybody with more than a passing interest in knives has certainly encountered the tired old controversy of forging versus stock removal. Although I am a forge guy myself, I have often admired the restraint my grinding brothers have shown while being told how inferior their product was because they hadn�t hammered on the steel. This is particularly ironic when one considers the amount of things that can go wrong in the process of forging, making the chances of getting a better knife from a grinder much greater! Nevertheless, there are few topics more rife with controversy than blade forging; this is mostly due to an abundance of wild speculation and gratuitous assumption by people who know little about what they are so boldly speaking of.
I once thought that the blade-making crowd was evolving at such a rate that the superior forged blade nonsense would soon disappear. With more folks having access to good information, and the popularity that forging has enjoyed, my hopes were that by now the craft could stand on its own legitimate merits without having to resort to the shameless old bogus claims. But the same fantastic stuff just keeps being repackaged for a next generations PR.
In response to this sad state, I offer the following paragraphs in hope of shedding enough light on this subject to eliminate some of the misconceptions once and for all, in a format that one would not need an engineering degree to understand. In researching these matters I have concluded that a major portion of science is simply the rewording of common sense and easy to understand general concepts into more precise and specific terms,you know- techno-babble, so I apologize in advance if some of this gets a little heavier than intended.
Why all the wishful thinking about forging? Stock removal consists of but a bar of steel, a grinder, and the will to remove everything that doesn�t look like a knife. Forging involves more output in tools, often a separate shop, plenty of time spent on hot, sweaty and imprecise work that involves burns and an unhealthy dose of respiratory hazards. Perhaps much of the bladesmith�s delusions of grandeur stems from a need to justify all the extra trouble. Another facet of the problem that cannot be ignored is what I like to refer to as �ancestor worship.� You know the routine - all modern products are junk because we either lack the motivation or the wisdom to do it like they did in ancient times. I must admit that in our modern �disposable� society that this can be true of many things but, with something as critical to a civilization as metal production, changes are seldom in the backwards direction. In ages past a smith did need to stand at an anvil for hours on end to produce even the raw materials but, as we will explore, for more reasons than expediency this is no longer the case. The old �they don�t make them like they used to� adage only goes so far. When gravely ill, how many of us would choose a 14th century barber over a 21st century surgeon?
My research has shown me that forging does make superior steel� from the cast ingot! Steel in the ingot, directly from the pouring process, requires heavy reduction and deformation in order to improve its properties by the working out of undesirable conditions, as in redistribution of brittle segregated constituents, closing up porosity and scattering any undesirable inclusions. Because of this, all traditionally poured steels undergo heavy rolling and other mill operations very soon after their creation. It is funny that if one looks at it like this, even stock removers use forged steel. It somewhat smacks of hubris to take a piece of steel that has alread been reduced from feet to fractions of an inch in thickness, hammer a bevel down one side and then proudly claim we have made the steel superior by our forging. Compared to these massive reduction operations, our meager hammering is little more than mostly repeated heat treating and, if one approaches it from the standpoint that if we use the recommended forging temperatures, then it truly does all come down to the heat treat.
Going against the grain
One undeniable aspect of forging things to shape, that we can get out of the way immediately, deals with the directional structure of steel from the aforementioned milling process. This condition is the result of the elongation of impurities, voids and inclusions in the direction of the rolling operation resulting in a wood grain type effect, such that the material will have slightly different properties in one direction than in another.
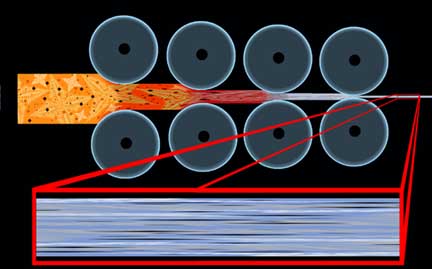 In order to avoid a very common confusion, it must be heavily stressed that the term �grain� in this case has nothing to do with the crystalline structure of the metal, such as an �austenite grain,� but instead refers to this directional property, as in �going against the grain,� and is not affected by annealing and other heat treatments. The condition where a property of a material is different in one direction than it is in another is known as �anisotropy,� and we will examine it again later in this discussion.
In order to avoid a very common confusion, it must be heavily stressed that the term �grain� in this case has nothing to do with the crystalline structure of the metal, such as an �austenite grain,� but instead refers to this directional property, as in �going against the grain,� and is not affected by annealing and other heat treatments. The condition where a property of a material is different in one direction than it is in another is known as �anisotropy,� and we will examine it again later in this discussion.
The quickest and easiest way to demonstrate how forging can affect the properties of a tool via this directional nature is in the classic crank shaft example:
 If one can imagine these cranks made of wood, they will quickly see the advantages of forging over stock removal in the strength of complex shapes; however, when we are talking shapes as simple as blades the effects would be hardly noticeable. As we will see later, claims that forging aligns �grain� with the edge of a blade are a sneaky play on words that can only effectively apply to this condition if the parent stock was carelessly sheared from a sheet perpendicular to its natural direction.
If one can imagine these cranks made of wood, they will quickly see the advantages of forging over stock removal in the strength of complex shapes; however, when we are talking shapes as simple as blades the effects would be hardly noticeable. As we will see later, claims that forging aligns �grain� with the edge of a blade are a sneaky play on words that can only effectively apply to this condition if the parent stock was carelessly sheared from a sheet perpendicular to its natural direction.
Demythifying the other mythconceptions
Excluding the minimal effects of the aforementioned directional condition, where does this idea of creating a superior blade by forging or making a better metal by hammering originate? It is a very old concept left over from the days when it was a fact. Before the industrial revolution gave us methods to mass produce molten steel at our bidding, there was the age old process of bloomery steel. A bloom of metal reduced from iron ore never actually reaches a completely liquid state but instead relies upon the chemistry of reduction to create a spongy mass of metal particles that is virtually useless until the smith hammers it solid and forges useable tools from it. For centuries forging wasn�t just a way to make better steel, it was the only way to make steel at all.
Another laurel for the forging process to falsely rest upon seems to revolve around cold working techniques, which are in opposition to most heat treating operations. Before heat treating, mankind relied upon cold hammering metals to make acceptable weapons by altering the properties of the materials. So this is a good place to start our discussion.
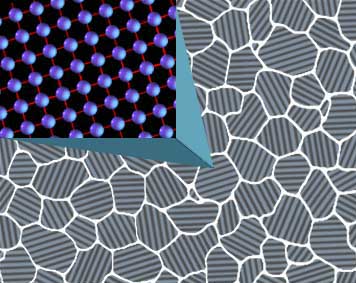 As a metal, steel is crystalline in nature, that is, it is made up of collections of atoms that are arranged in an orderly and repeating pattern. Each individual crystal within the metal has a different lattice work orientation to distinguish it from its neighbors. For illustrative purposes the image to the right shows this as a spaced grid work with lines but, in actuality, try to imagine atoms stacked like ping pong balls or oranges on a fruit stand; ordered rows and layers making an entire stack.
As a metal, steel is crystalline in nature, that is, it is made up of collections of atoms that are arranged in an orderly and repeating pattern. Each individual crystal within the metal has a different lattice work orientation to distinguish it from its neighbors. For illustrative purposes the image to the right shows this as a spaced grid work with lines but, in actuality, try to imagine atoms stacked like ping pong balls or oranges on a fruit stand; ordered rows and layers making an entire stack.
Much of the bad information stems from a gross misunderstanding of the basic process of rearranging these stacks. From impossible suggestions of shrinking matter, to misplaced desires of �breaking� up the internal structures, much of the bladesmith�s claims bear little resemblance to physical fact. The basic law of conservation of matter states that the mass of a system of substances is constant; simple junior high science should have taught us that shrinking existing steel grains is simply not an option. If anybody manages to produce a smaller blade with the same mass as the parent bar, they need to rewrite all the laws that govern our universe. Equally misguided, the smith that aims to break up or fracture internal structures is not doing his blades any favors; while transgranular fracturing is possible, it is very bad.
Going back to the aforementioned laws of conservation, it is fundamental that while matter can neither be created nor destroyed, it can be rearranged. While so many of the bladesmith's fantasies are physically impossible, hammering steel can have its effects all the same by rearranging things in very profound ways. How conditions now drastically change from anything like a fruit stand is that oranges can be squeezed, metal atoms cannot. The atomic stacking of steel, within the context of this article, cannot be condensed tighter, and that is why forging works the way it does. A ball of clay is much softer than steel, but they deform in a similar fashion. If we place that clay in a cylinder and bring a tight sealed piston to bear upon it, no amount of force we could muster would force the piston down beyond the mass of our soft clay. Why should we imagine our stronger steel to be any different? But this stubbornness about being squeezed is a good thing for the process of forging. Be it clay or steel, if we pinch it in one direction it will expand in another; this is how forging steel works and why "packing" steel cannot. If we are going to change the shape of steel we must find a way more in accordance with reality than compacting iron atoms. If we can't get those atoms to squeeze a little closer to accommodate our fantasies, perhaps we can get them to move around each other to achieve our goals, and that is what we will now examine.
Moving the metal
Let us establish some terms, the first of which will be deformation. When I say �deformation� in this article, I am referring to a change of original shape for the metal. I will discuss two types of deformation - elastic and plastic. Elastic deformation is a change of shape that is entirely reversible upon removal of the effecting load. A good example of this would be flexing a blade and then having it return to true. Rubber has plenty of elasticity, while steel has much less. Inversely, plastic deformation would be a shape change that is permanent after the load is removed, as in bending a blade and having it stay bent. Plasticity (or ductility as it is described in metals) is very high in clay and much less in steel.
A term you will often see in dealing with metals is �yield� point. Each metal has its own range of elasticity before it yields and permanently (plastically) deforms. This all occurs by movement of the rows of atoms within the metal. Think of our stack of ping pong balls; if you wish to move an entire row or layer you need to push from the side until all of the individual balls will move from their niches in the stacking. If you only push far enough for the top row to move slightly out of their nooks and then back off to let them return to their original positions, you have now duplicated elastic deformation in metal. If you imagine the process occurring over millions of rows of atoms, this would be flexing the steel.
On this stacked atomic level plastic deformation, or forging as we know it, occurs by a mechanism known as slip; the name describes it all. If you keep pushing until the ping pong balls slip into the next niche over, they will remain in that position when the pressure is released and you have now permanently (plastically) deformed the stack of ping pong balls. Translate this to millions of layers of iron atoms and you get a bent piece of steel. As you may have guessed, this is why the process by which metal deforms is called �slip;� it is literally millions of rows of iron atoms slipping over each other.
Slip occurs on optimal planes determined by the stacked arrangement of the atoms (our oranges or ping pong balls). It is easiest to get the layers to move along the direction of tightest stacking. To get a better grasp of this, think of gluing our ping pong balls to two boards in a widely spaced pattern. Put the boards together, ping pong balls to ping pong balls, and begin to tilt the bottom board. You will have to go to a very steep angle before the top board will slip from its position of the nestled ping pong balls. Now do the experiment again, but this time gluing the balls very close together. The top board will now slip at a much lesser angle.
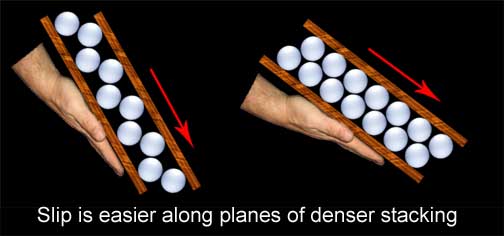 Due to the ordered stacking of iron atoms, there are preferred directions and angles in which slip will occur and others that it will not. Let�s now imagine a single iron crystal as a deck of cards. Push straight down and the deck will be unchanged; put it on edge and it still will not move, but just nudge the side on an angle and the deck will deform and rotate in the direction of push. Each individual crystal in steel can only deform along certain planes but, since the metal is made up of many crystals with a different orientation, some grains will be at the optimal angle for slip to occur and others will not. Those others will be pulled along until the rotation of their orientation offers those choice planes, allowing the metal to deform as a whole in almost any direction.
Due to the ordered stacking of iron atoms, there are preferred directions and angles in which slip will occur and others that it will not. Let�s now imagine a single iron crystal as a deck of cards. Push straight down and the deck will be unchanged; put it on edge and it still will not move, but just nudge the side on an angle and the deck will deform and rotate in the direction of push. Each individual crystal in steel can only deform along certain planes but, since the metal is made up of many crystals with a different orientation, some grains will be at the optimal angle for slip to occur and others will not. Those others will be pulled along until the rotation of their orientation offers those choice planes, allowing the metal to deform as a whole in almost any direction.
Handy little allotropes
Each metal has a particular atomic arrangement at room temperature and it is this that gives the metal its working properties and strengths. One stacking arrangement is called face centered cubic (fcc). Fcc offers very tight stacking at very convenient angles so it is quite conducive to slip. Gold is fcc at room temperature and hence it has most desirable working properties.
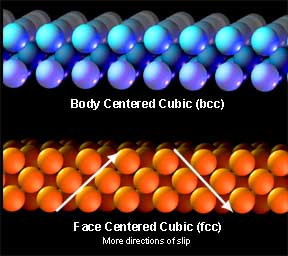 Another atomic arrangement is body centered cubic (bcc). Bcc offers larger niches for atoms to rest in and slip has to occur at very odd angles, so metals with this configuration are less ductile, with more elastic deformation than plastic deformation. Iron is bcc at room temperature so it is more difficult to work than gold. But iron has a convenient ability to change stacking arrangements when heated. If you get iron hot enough it will change to fcc, the same arrangement as gold at room temperature. Don�t tell the alchemists that we smiths had it figured out a long, long time ago!
Another atomic arrangement is body centered cubic (bcc). Bcc offers larger niches for atoms to rest in and slip has to occur at very odd angles, so metals with this configuration are less ductile, with more elastic deformation than plastic deformation. Iron is bcc at room temperature so it is more difficult to work than gold. But iron has a convenient ability to change stacking arrangements when heated. If you get iron hot enough it will change to fcc, the same arrangement as gold at room temperature. Don�t tell the alchemists that we smiths had it figured out a long, long time ago!
But since iron is normally bcc, working below the critical temperature is slow going and even at 1000�F. can still be called �cold� working since the rate of deformation far exceeds the rate of recrystalization (but we will discuss that some more later).
From the atomic lattice to the anvil�s face
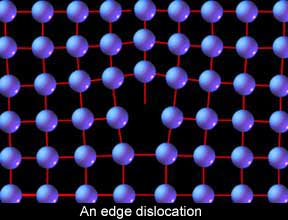 Let�s put this all together and start looking at moving metal. First let's examine how steel moves even when cold. When scientists first started to understand this process, they examined the nature of the atomic stacking in crystals, crunched the numbers, and encountered some problems. If metals were made up of all these perfect, repeating rows of atoms, it should take as much as a thousand times more force to initiate the slip process than what it actually does. Something wasn�t right. So they took a closer look and found the source of the discrepancy. The metal crystals are not perfect but have many little inconsistencies built into them in the form of �vacancies� and �dislocations.� Vacancies are exactly that, a space in the stacking that is empty where an atom should be. For our discussion, however, dislocations are of greater interest, particularly �edge dislocations.� Edge dislocations are a deviation in the stacking where one row is incomplete and causes an odd distortion in the lattice.
Let�s put this all together and start looking at moving metal. First let's examine how steel moves even when cold. When scientists first started to understand this process, they examined the nature of the atomic stacking in crystals, crunched the numbers, and encountered some problems. If metals were made up of all these perfect, repeating rows of atoms, it should take as much as a thousand times more force to initiate the slip process than what it actually does. Something wasn�t right. So they took a closer look and found the source of the discrepancy. The metal crystals are not perfect but have many little inconsistencies built into them in the form of �vacancies� and �dislocations.� Vacancies are exactly that, a space in the stacking that is empty where an atom should be. For our discussion, however, dislocations are of greater interest, particularly �edge dislocations.� Edge dislocations are a deviation in the stacking where one row is incomplete and causes an odd distortion in the lattice.
 Why are edge dislocations of such interest to us? Because they are responsible for the ability of the humble human arm to move steel. Imagine an enormous area rug that is so heavy one man cannot drag it across the floor, and yet you need to adjust its position slightly. How can it be done? Make a wrinkle or roll in one end and then, like an inch worm, simply chase the roll to the other end! This is how edge dislocations facilitate slip in steel.
Why are edge dislocations of such interest to us? Because they are responsible for the ability of the humble human arm to move steel. Imagine an enormous area rug that is so heavy one man cannot drag it across the floor, and yet you need to adjust its position slightly. How can it be done? Make a wrinkle or roll in one end and then, like an inch worm, simply chase the roll to the other end! This is how edge dislocations facilitate slip in steel.
However, edge dislocations only make deformation easier until they multiply and accumulate against obstacles like grain boundaries, disproportionate alloying atoms, or other inconsistencies in the stacking. As these pile up, they store the kinetic energy of deformation in potential energy known as strain. In yet another case of extreme irony, as opposed to the utterly false claims of edge packers, fond of low temperature working, reality shows us that cold worked steel is actually less dense, on a small scale, due to increased vacancies and dislocations. All of this is the basis of work hardening and is very important information for our discussion.
To help in understanding work hardening of metals, I like to visualize a metal crystal as a large cube of blocks made up of hundreds of little blocks all neatly stacked in orderly rows and columns. Choose a random point anywhere in the stack and give one of the rows a push until they move out of alignment. Now choose another side of the stack and do it again. If you continue doing this, the misaligned rows are going to start tangling with each other and it is going to get harder to find a row that will easily slide. Eventually enough misaligned jams will form so that the pile, while not the same original shape at all, will become quite rigid in its new shape. If you continued to push harder without movement, you may succeed in tumbling an entire portion of your stack, and I think we can all guess what this would equate to in our steel!
Strain hardening leads to an anisotropy that is only beneficial, from a strengthening standpoint, in an orientation parallel to the direction of cold working. The opposite applies to the properties in a direction perpendicular to that working. In order to get properties in all directions (i.e., isotropy), a stress-relieving operation will most likely be necessary to re-obtain that uniformity. When discussing the nature of strain hardening it is also worth mentioning that, while ductile strength yield point and hardness are increased, the scratch hardness or the difficulty of cutting or machining is not.
But if we wish to continue to work our metal more, when further force will only result in it coming apart, how do we proceed? Cold metal workers stumbled upon the answer millennia ago and have used it ever since; if bladesmiths are a little slow to catch on, it is because so much of our work already incorporates it at our preferred temperatures.
Annealing
Annealing, is the process of heating cold worked and other strain-laden metals to a temperature at which a number of important things will happen. The first is known as recovery, this is the point in heating where the strain energy will be relieved and the dislocations will be reduced as the lattice relaxes. The next step, when the atomic arrangement begins the change known as recrystalization, will result in entirely new crystals forming to replace the old deformed ones. This process will also occur in steps beginning with nucleation of the new grains at points of highest strain energy, which are usually at the old boundaries and other distortions in the lattice.
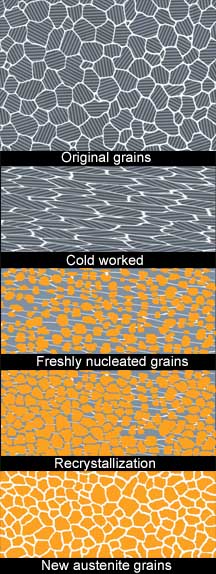 In fact many simple metals, those lacking inherent sources of high strain energy, actually require deformation in order to recrystalize upon subsequent reheating. A misinterpretation of this concept may very well be the source of some of the false notions we have about improving steel with hammering. You see, steel is not a simple metal; it is very complex with all kinds of inconsistencies built right into its lattice. Steel can recrystallize every time it is heated to the appropriate temperature with no help at all from the hammer, although hammering can add to the turmoil.
In fact many simple metals, those lacking inherent sources of high strain energy, actually require deformation in order to recrystalize upon subsequent reheating. A misinterpretation of this concept may very well be the source of some of the false notions we have about improving steel with hammering. You see, steel is not a simple metal; it is very complex with all kinds of inconsistencies built right into its lattice. Steel can recrystallize every time it is heated to the appropriate temperature with no help at all from the hammer, although hammering can add to the turmoil.
With all this in mind, one needs to reexamine the claims of mechanically refined conditions at any temperature while remembering that any effects from plastic deformation will be rearranged as soon as the metal is heated to anywhere near recrystalization by the very process that makes stress relieving and annealing possible. To be entirely successful, quench hardening heat treatment requires those same processes. Many of the claims of improvements via deformation can be in direct opposition to proper heat treating.
As we have already discussed, steel will form all new crystals when heated above the recrystallization temperature, and that hammering can add points for this process to occur, but what happens if you hammer at a temperature beyond the recrystallization point? Each hammer blow will introduce energy into a system that is trying to equalize itself; this new energy will then initiate fresh nucleation points and more new grains. Proper forging temperatures are a balance of the need to move the metal and keeping pace with the rate of grain growth. But for all of the frustrations steel can give us, it has some wonderful safety mechanisms built into it that can save the work of even the most incompetent smith.
Since steel has the ability to recrystallize just fine all on its own, a process called �normalizing� becomes very significant to bladesmiths. Quick heating to the recrystallization temperature, while just leaving the poor metal alone to do its thing, will wipe the slate clean, even out all of the problems our inconsistent pounding can bring, and refine the grain better than any of our magic hammer taps.
But what about forging refining the grain? Isn�t that what bladesmiths do? Yes, but a wise smith with the knowledge of what steel is really capable of will get out of its way and let it fix itself much more efficiently. If finer grain is superior for our purposes (and in most cases it is), then why only have finer grain in just the spots our uneven hammer blows managed to catch? Why not relax and let the heated steel do the work and make the entire blade uniformly fine?
Any time I have used various thermal treatments alone to refine grain size, and then compared the fractured grain appearance to samples that I had hammered into submission, the former were always smoother and more uniform in appearance. This should come as no surprise when one considers how complete or uniform one�s hammer blows could be when compared to the action of the entire internal structure of the steel at work. Also worth mentioning is an interesting phenomenon which metallurgists have observed, and bladesmiths need to consider. If steel is only lightly deformed, there will be enough uneven points of nucleation created for many little grains to form around a few large grains. Since large grains grow at the expense of smaller grains, what we have done in this circumstance is created a monster and surrounded it with all it can eat. Overall, this would result in an uneven grain coarsening effect, which one would not have if they had just let the steel do its thing, or at least really moved the steel enough to totally rework it. This would render the idea of �packing� with a few light blows at the end completely counterproductive.
What does it all mean?
In conclusion, after examining all of these facets of a complex process, there are indeed effects that forging can have upon the metal, however, much of it hardly falls into the realm of the fantastic claims that too many bladesmiths prop themselves up on a pedestal with, and the majority of these claims are easily debunked with an elementary understanding of the actual mechanisms at work. Too often the reason bladesmiths need extra ways to refine the steel is in order to fix all the extra abuse they heap upon it in forging it, another one of many odd �Catch-22�s� in the business. The fact is that, unless we just cold work it all and call it good, everything relies upon heating to those temperatures necessary to accomplish any of it. So if we let go of the concept of a hammer doubling as some sort of magic wand, tapping miraculous changes into the steel, and just approach the forging process as another step in heat treatment it could probably make a pretty good blade.
Tags:
Replies to This Discussion
Events
-
2014 is the Chinese Year of the Horse
February 17, 2026 at 12am to February 5, 2027 at 12am – where & how you choose
Birthdays
Birthdays Today
Birthdays Tomorrow
Important (read & understand)
Skype: Travelingraggyman
Email and Instant Messenger:
TravelerinBDFSM @ aol/aim; hotmail; identi.ca; live & yahoo
OR
Travelingraggyman @ gmail and icq ***

1AWARD UPDATES & INFORMATION
10,000 votes - Platinum Award
5,000 votes - Gold Award
2,500 votes - Silver Award
1,000 votes - Bronze Award
300 votes - Pewter Award
100 votes - Copper Award
Member of the Associated Posting System {APS}
This allows members on various sites to share information between sites and by providing a by line with the original source it credits the author with the creation.
Legal Disclaimer
***************We here at Traveling within the World are not responsible for anything posted by individual members. While the actions of one member do not reflect the intentions of the entire social network or the Network Creator, we do ask that you use good judgment when posting. If something is considered to be inappropriate it will be removed
Site Meter
This site is strictly an artist operational fan publication, no copyright infringement intended
Patchwork Merchant Mercenaries had its humble beginnings as an idea of a few artisans and craftsmen who enjoy performing with live steel fighting. As well as a patchwork quilt tent canvas. Most had prior military experience hence the name.
Patchwork Merchant Mercenaries.
Vendertainers that brought many things to a show and are know for helping out where ever they can.
As well as being a place where the older hand made items could be found made by them and enjoyed by all.
We expanded over the years to become well known at what we do. Now we represent over 100 artisans and craftsman that are well known in their venues and some just starting out. Some of their works have been premiered in TV, stage and movies on a regular basis.
Specializing in Medieval, Goth , Stage Film, BDFSM and Practitioner.
Patchwork Merchant Mercenaries a Dept of, Ask For IT was started by artists and former military veterans, and sword fighters, representing over 100 artisans, one who made his living traveling from fair to festival vending medieval wares. The majority of his customers are re-enactors, SCAdians and the like, looking to build their kit with period clothing, feast gear, adornments, etc.
Likewise, it is typical for these history-lovers to peruse the tent (aka mobile store front) and, upon finding something that pleases the eye, ask "Is this period?"
A deceitful query!! This is not a yes or no question. One must have a damn good understanding of European history (at least) from the fall of Rome to the mid-1600's to properly answer. Taking into account, also, the culture in which the querent is dressed is vitally important. You see, though it may be well within medieval period, it would be strange to see a Viking wearing a Caftan...or is it?
After a festival's time of answering weighty questions such as these, I'd sleep like a log! Only a mad man could possibly remember the place and time for each piece of kitchen ware, weaponry, cloth, and chain within a span of 1,000 years!! Surely there must be an easier way, a place where he could post all this knowledge...
Traveling Within The World is meant to be such a place. A place for all of these artists to keep in touch and directly interact with their fellow geeks and re-enactment hobbyists, their clientele.
© 2025 Created by Rev. Allen M. Drago ~ Traveler.
Powered by
![]()