Traveling within the World
Linking your favorite traveling artists across the globe
Basic Metallurgy by Kevin R. Cashen, www.cashenblades.com
I have assembled this page as an introduction to some of the basic metallurgy involved in bladesmithing. The starting point of any blade is steel, an alloy of iron with a little carbon added, but iron has an interesting property which allows bladesmiths to do extraordinary things with it.
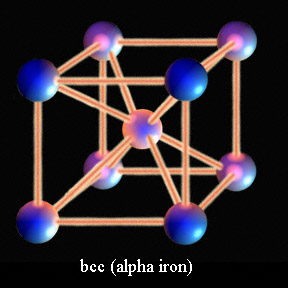
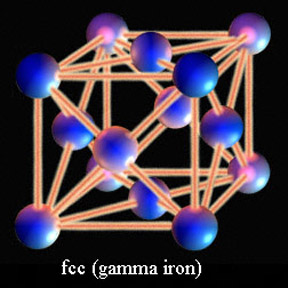 Iron is able to exist in different phases, or atomic stacking, based upon its temperature. At room temperature iron is crystalline structure with its atoms stacked in a cubic arrangement with an extra atom in the center. This body centered cubic (bcc) iron is known as alpha iron and only has so many spaces to accommodate carbon atoms between the iron. At temperatures above 1335 degrees Fahrenheit the atomic stacking of iron changes to a cube with an extra iron atom on the middle of each face of that cube. This face centered cubic phase is known as gamma iron, and it has many more spaces for carbon to rest between the iron than room temperature alpha iron.
Iron is able to exist in different phases, or atomic stacking, based upon its temperature. At room temperature iron is crystalline structure with its atoms stacked in a cubic arrangement with an extra atom in the center. This body centered cubic (bcc) iron is known as alpha iron and only has so many spaces to accommodate carbon atoms between the iron. At temperatures above 1335 degrees Fahrenheit the atomic stacking of iron changes to a cube with an extra iron atom on the middle of each face of that cube. This face centered cubic phase is known as gamma iron, and it has many more spaces for carbon to rest between the iron than room temperature alpha iron.

The predominantly iron portion of steel. Since it holds relatively small amounts of carbon proeutectoid ferrite, like in the image to the left, occurs in alloys with less than .80% carbon.

Cementite or iron carbide (Fe3C) is the form that carbon takes in steel, cast iron and other iron-carbon alloys. It is a compound of 93.33% iron and 6.67% carbon by weight, but occurs in alloys, in the form of proeutectoid cementite, above .83%C. Pictured is cementite (white) in the grain boundaries of 1095 steel, not a desirable condition.

Named for Sir W.C. Roberts Austen this is a solid solution structure in which gamma iron is the solvent with carbon or iron carbide as the solute. Austenite is the state of iron/carbon that most of the structures (martensite, pearlite, bainite etc...) used in bladesmithing are derived from. Upon heating to the temperature designated ac1 alpha iron makes the allotropic shift from Body centered cubic to face centered cubic gamma iron. Gamma iron is capable of holding much more carbon in solution and begins to accept carbon into the iron atomic matrix. Holding higher amounts of carbon in solution in the FCC configuration causes austenite to be unstable at temperatures below ar1. Upon slow cooling carbon will diffuse to form pearlite from the parent austenite. If rapidly cooled austenite will be unable to diffuse carbon sufficiently enough to form pearlite and the result will be martensite or bainite depending upon the rate of cooling. In many ways austenite is the parent of the other microstructures, not only from the standpoint that the other structures arise from its decomposition but also that it provides the framework for some of their characteristics. Austenite leaves its affects and "finger print" in the form of the austenite grain boundary. Shape and size of the austenite grain will determine the rates of transformation through points of nucleation, or "toe holds" for transformations to begin. And this in turn will affect the formation of new austenite upon reheating to ac1. Steel with larger austenite grains tends to harden more deeply due to the reduction of nucleation points for the diffusion of pearlite to begin. The drawback is that larger grains make steel much more weak and brittle. For bladesmithing the greater of these two evils is the brittleness so large grains and grain growth is to be avoided whenever possible. Austenite grain growth occurs when the steel is heated beyond ac1 and Acm (the point at which the extra cementite is dissolved), and increases with temperature. The larger austenite grains will grow at the expense of the smaller grains. So time at these elevated temperatures should be carefully watched and kept to a minimum. When heating steel to ac1 the shift to gamma iron will allow any pearlite to be dissolved and form new austenite grains. These new grains will be slightly finer and within the previous boundaries due to increased nucleation. As temperature increases the proeutectoid ferrite (if the steel is hypoeutectoid) or proeutectoid cementite (if the steel is hypereutectoid) will be dissolved until ac3 or Acm and there is complete austenite.
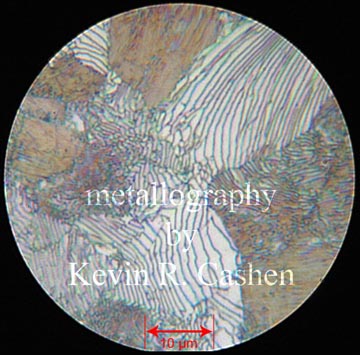
When steel that has been heated until carbon is in solution is then allowed to cool slowly that carbon will come out of solution in localized areas of sheet like banding, or lamellae. These lamellae will then create zones of carbon depleted ferrite on either side and this pattern will repeat throughout the prior austenite grains. The lamellae will scatter the light of an optical microscope creating an odd iridescent effect that resembled mother of pearl, hence the name of this steel phase. With the carbon our of solution in this manner pearlitic steel is much softer and becomes more ductile and malleable, however in steel will carbon in excess of .8% the carbide sheets can grow very large and in some undesirable places causing difficulties in machining and embrittlement. For this reason the old standard bladesmith annealing by heating above critical and then slow cooling by insulating may work fine for medium carbon steels, but can be complicated by higher carbon alloys. For this reason, instead of the lamellar method, I use a different annealing that is commonly used in the steel industry for high carbon steel.

When steel is heated to temperatures below the critical the carbon is permitted to move but to a much lesser degree than higher temperatures, this causes the carbon to form spheroidal carbides in many fine localized points within the soft ferrite matrix. The heat treating process for this is called spheroidizing and is a very good way to get high carbon steel very soft. The spheroidal carbides offer much less resistance to cutting tools than the sheets of carbide in lamellar pearlite. Also added benefits of this approach to annealing are no affecting of grain size or other issues associated with heavy heating, but the grain and carbide condition must be carefully set up before this with proper normalizing.

Named for the German metallurgist Adolph Martens, Martensite is the hardened phase of steel that is obtained by cooling Austenite fast enough to trap carbon atoms within the cubic iron matrix distorting it into a body centered tetragonal structure. Since its formation is accomplished by quick cooling, avoiding the formation of pearlite, it is a sheer dependent diffusionless transformation. Free of diffusion processes, it has the same composition as the parent austenite. Under the microscope in cross section it appears acicular, or needle like, but in 3 dimensions is actually either lath or plate in structure. Alloys with less than .6 percent carbon form lath martensite. Alloys with more than 1 percent carbon form plate martensite and alloys with from .6 to 1 percent carbon form mixtures of the two in varying degrees. The temperature at which martensite begins to form in an alloy is given the designation Ms (martensite start). Formation occurs in "packets" with lath martensite, as the crystalline structure begins with single plane forming across the grain with many subsequent branches nucleating from the central one in a parallel "fern' or "feather" like configuration. Plate martensites form in larger plates that have a tendency to approach others at varying angles (irrational habit planes). Points of great stress are created where the plates intersect, causing plate martensite to be more brittle.
Tags:
Replies to This Discussion
Events
-
2014 is the Chinese Year of the Horse
February 17, 2026 at 12am to February 5, 2027 at 12am – where & how you choose
Birthdays
Birthdays Today
Birthdays Tomorrow
Important (read & understand)
Skype: Travelingraggyman
Email and Instant Messenger:
TravelerinBDFSM @ aol/aim; hotmail; identi.ca; live & yahoo
OR
Travelingraggyman @ gmail and icq ***

1AWARD UPDATES & INFORMATION
10,000 votes - Platinum Award
5,000 votes - Gold Award
2,500 votes - Silver Award
1,000 votes - Bronze Award
300 votes - Pewter Award
100 votes - Copper Award
Member of the Associated Posting System {APS}
This allows members on various sites to share information between sites and by providing a by line with the original source it credits the author with the creation.
Legal Disclaimer
***************We here at Traveling within the World are not responsible for anything posted by individual members. While the actions of one member do not reflect the intentions of the entire social network or the Network Creator, we do ask that you use good judgment when posting. If something is considered to be inappropriate it will be removed
Site Meter
This site is strictly an artist operational fan publication, no copyright infringement intended
Patchwork Merchant Mercenaries had its humble beginnings as an idea of a few artisans and craftsmen who enjoy performing with live steel fighting. As well as a patchwork quilt tent canvas. Most had prior military experience hence the name.
Patchwork Merchant Mercenaries.
Vendertainers that brought many things to a show and are know for helping out where ever they can.
As well as being a place where the older hand made items could be found made by them and enjoyed by all.
We expanded over the years to become well known at what we do. Now we represent over 100 artisans and craftsman that are well known in their venues and some just starting out. Some of their works have been premiered in TV, stage and movies on a regular basis.
Specializing in Medieval, Goth , Stage Film, BDFSM and Practitioner.
Patchwork Merchant Mercenaries a Dept of, Ask For IT was started by artists and former military veterans, and sword fighters, representing over 100 artisans, one who made his living traveling from fair to festival vending medieval wares. The majority of his customers are re-enactors, SCAdians and the like, looking to build their kit with period clothing, feast gear, adornments, etc.
Likewise, it is typical for these history-lovers to peruse the tent (aka mobile store front) and, upon finding something that pleases the eye, ask "Is this period?"
A deceitful query!! This is not a yes or no question. One must have a damn good understanding of European history (at least) from the fall of Rome to the mid-1600's to properly answer. Taking into account, also, the culture in which the querent is dressed is vitally important. You see, though it may be well within medieval period, it would be strange to see a Viking wearing a Caftan...or is it?
After a festival's time of answering weighty questions such as these, I'd sleep like a log! Only a mad man could possibly remember the place and time for each piece of kitchen ware, weaponry, cloth, and chain within a span of 1,000 years!! Surely there must be an easier way, a place where he could post all this knowledge...
Traveling Within The World is meant to be such a place. A place for all of these artists to keep in touch and directly interact with their fellow geeks and re-enactment hobbyists, their clientele.
© 2025 Created by Rev. Allen M. Drago ~ Traveler.
Powered by
![]()